Welcome to Clinical Trials
We invite you to participate in one of our clincial trials; to increase your knowledge of your condition and to help future generations

- Home
- Participants
Participants
What is a Clinical trial?
A clinical trial is a process that observes and studies the effect of a treatment, medicine or medical device, when they are administered to selected groups of people. Clinical trials are carefully designed and approved by an Independent Ethics Committee (IEC) before they can begin the trial on participants.
Newly developed and discovered medicines must be tested through clinical trials before they are approved for use. The goal of a clinical trial is to determine if a medicine is safe and what is the most effective dose of that medicine.
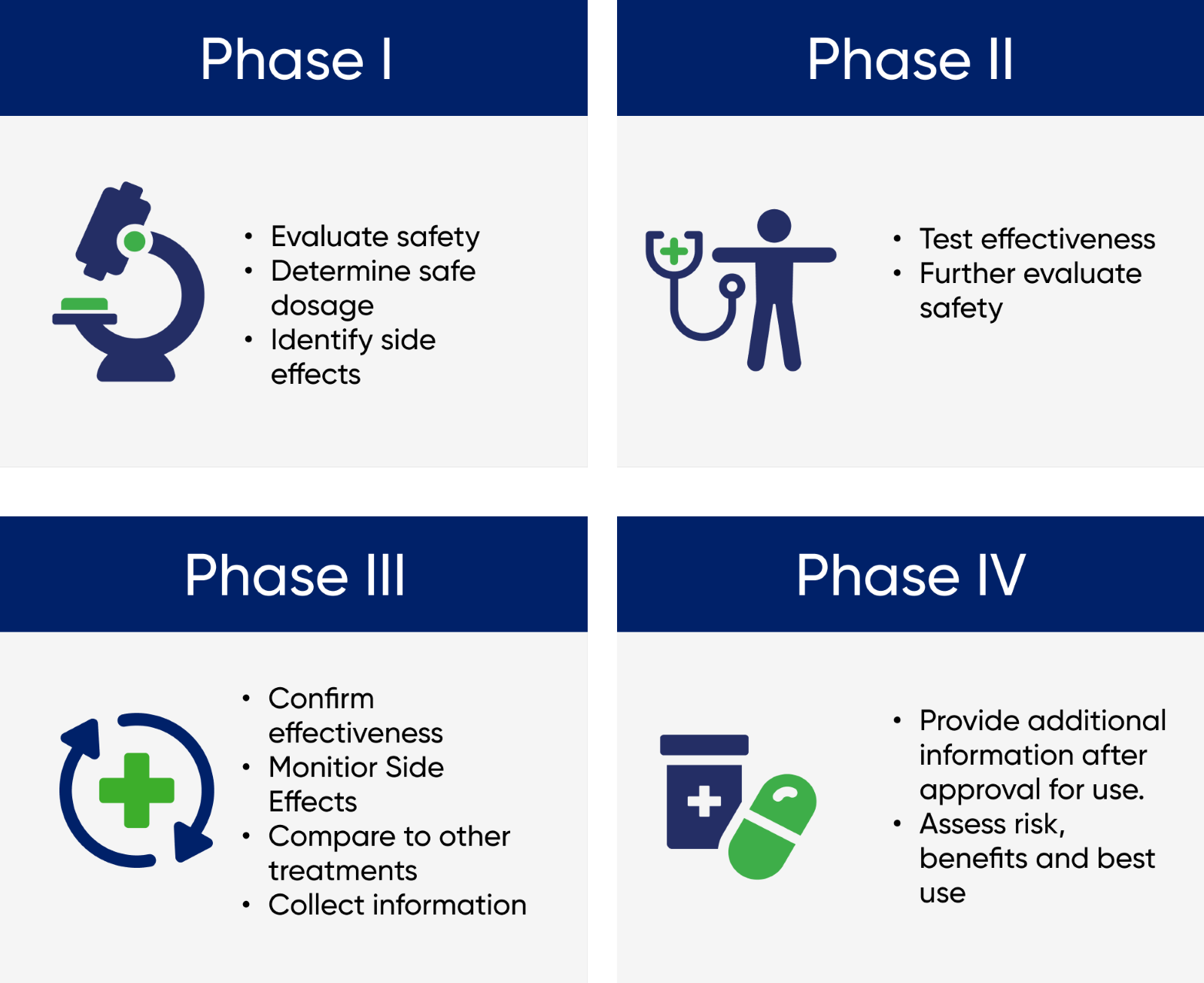
There are the four phases of clinical trials and what do they mean?
Early phase trials examine whether a drug is safe on humans and what side effects it might have, later phase trials will need to prove the correct dosage required for it to be effective, and study the long term effects of use.
How do I find a clinical trial that’s right for me
You can check if you are eligible for any of our currently recruiting trials on our 'Recruiting Trials' page, and/or register your interest for current or future studies. Alternatively, for more general participant enquiries, if you apply via our contact form one of the ACTT team will contact you directly.
You can ask your GP or your clinical specialist for more information on clinical trials and if any of the currently recruiting trials would be of benefit to you. The 'Recruiting Trials' page provides the names and types of trials that you can then discuss with us or your GP.
Is there more information available?
Yes, we have a patient information brochure that is available for anyone to download. This outlines the process and offers more detail.
Participant Information Brochure
How to get involved in a Clinical Trial
Register Your Interest
Initially go to our Recuiting Trials page. Review trials by category and then see current trial listings in your area of interest. Each trial allows online expression of interest and in some cases self assessment is also available via an online questionnaire.
Pre Screening
Once you have applied online one of our coordinators will call you to discuss if you’re eligible & schedule an in person appointment
Provide Consent
After an in person consultation at Aotearoa Clinical Trials, if we all agree the trial is right for you then the next step is to provide written consent to participate.
Participation
Once you are accepted into a trial you are provided with full contact and appointment information for your visits, and all medical requirements are provided free of charge. Your participation now will ultimately then help future generations
Any other Questions
You can send an online enquiry or submit an expression of interest at any time via our contact us form
Find a clinical trial today
View our recruiting trials page to see if any of our current trials are right for you
Key Benefits Include
Participants in a clinical trial are closely monitored at no cost by a team of clinicians to protect your safety, improve treatment outcomes and assure data integrity. This offers the benefit of a deeper level of involvement in your own treatment which itself may give you improved health outcomes.
Clinicians conducting clinical trials are the leading experts in their field. Just by participating in a clinical trial the participant gains free access at no cost to the best doctors.
- Research clinicians are thought to be 5 years ahead of non-research clinicians
People who participate in a clinical trial gain access to the very latest innovative new medicines and clinical devices, sometimes years before they become available to the general public. When you hear about a remarkable new drug, you'll find that it was in fact introduced to patients years earlier.
In NZ, medicines are purchased by the Government drug buying agency PHARMAC. PHARMAC has a limited budget meaning the only way to access some innovative and new treatments is through clinical trials.
Clinical Trials have been shown in published medical studies to deliver superior health outcomes over standard care.
While there are many reasons for this the main ones appear to be:
- Participants have a detailed and documented outcome through their journey
- Participants develop a greater knowledge of their own disease
- Physicians involved in research appear to practice medicine in advance of their colleagues
- Increased interactions and face to face with top medical staff